Post-inhalation cough with therapeutic aerosols: Formulation considerations - ScienceDirect
Par un écrivain mystérieux
Last updated 08 juillet 2024

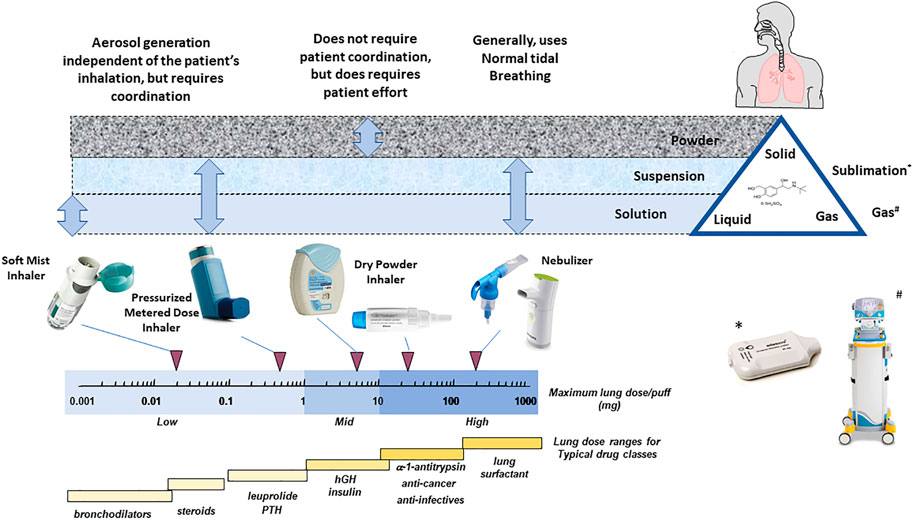
Frontiers Half a Century of Technological Advances in Pulmonary Drug Delivery: A Personal Perspective

PDF) Challenges for pulmonary delivery of high powder doses

Contemporary Formulation Development for Inhaled Pharmaceuticals - Journal of Pharmaceutical Sciences

PDF) Effect of a spacer on total systemic and lung bioavailability in healthy volunteers and in vitro performance of the Symbicort ® (budesonide/formoterol) pressurized metered dose inhaler

Novel dry powder inhaler formulation containing antibiotic using combined technology to improve aerodynamic properties - ScienceDirect

DDL2019 Digital Proceedings by info-ddl-conference - Issuu
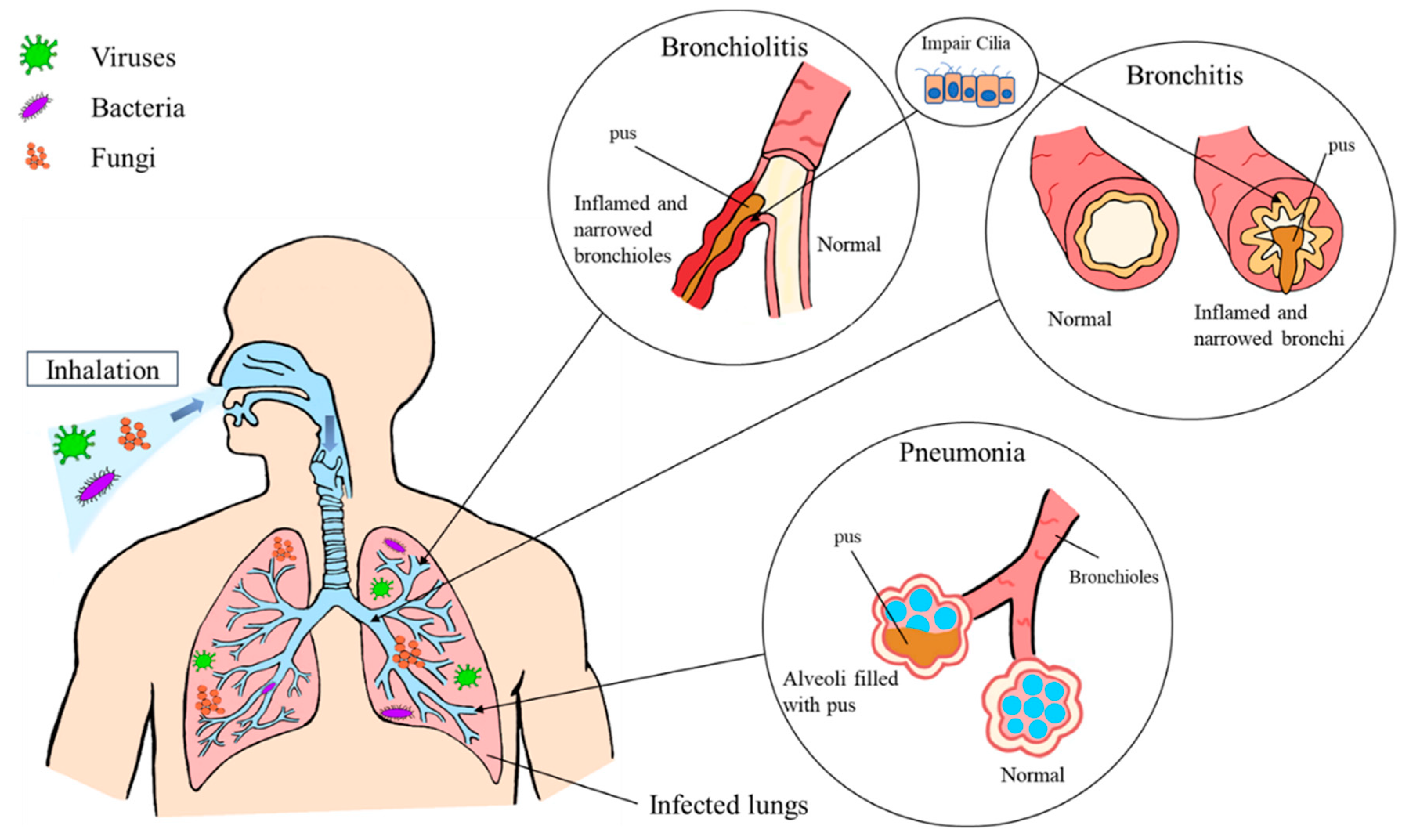
Pharmaceutics, Free Full-Text

Dry Powder Inhaler with the technical and practical obstacles, and forthcoming platform strategies - ScienceDirect
Essentials in saline pharmacology for nasal or respiratory hygiene in times of COVID-19
Recommandé pour vous
 Inhalation contre le rhume14 Jul 2023
Inhalation contre le rhume14 Jul 2023 Balsolène inhalation Rhume - Fumigation Nez bouché, sinusite, rhino14 Jul 2023
Balsolène inhalation Rhume - Fumigation Nez bouché, sinusite, rhino14 Jul 2023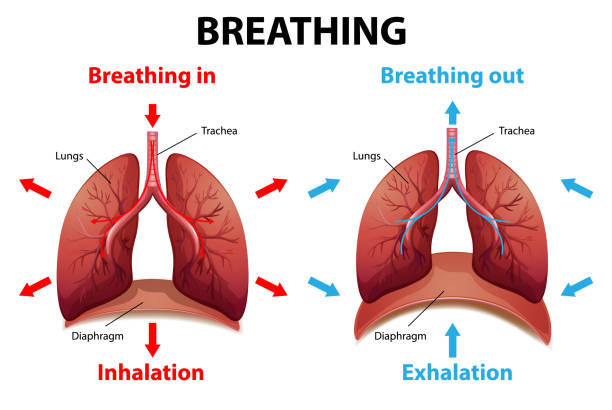 The Process Of Breathing Explained Stock Illustration - Download Image Now - Exhaling, Smoke Inhalation, Lung - iStock14 Jul 2023
The Process Of Breathing Explained Stock Illustration - Download Image Now - Exhaling, Smoke Inhalation, Lung - iStock14 Jul 2023- Biology Physics Chemistry - What is the difference between Inhalation and Exhalation? • Inhalation is the intake of air into lungs, whereas exhalation is the pushing out of air from lungs. •14 Jul 2023
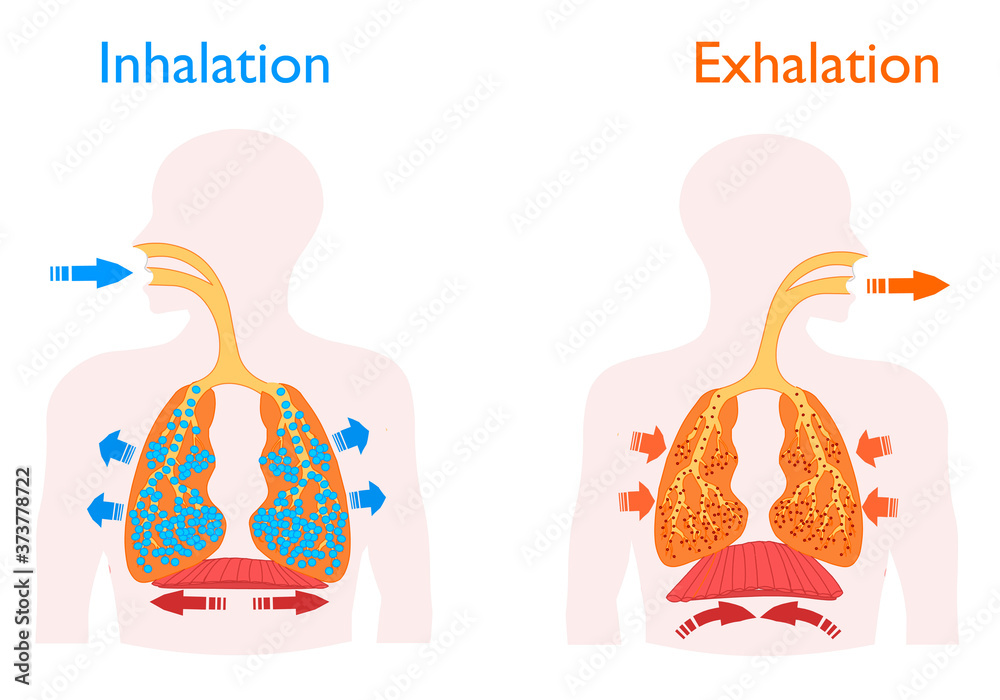 Inhalation exhalation. Human Breathing. The motion of the lung and diaphragm in inspiration, expiration. Blue arrow oxygen inlet, red carbon dioxide outlet. Pulmonary anatomy. Vector illustration Stock Vector14 Jul 2023
Inhalation exhalation. Human Breathing. The motion of the lung and diaphragm in inspiration, expiration. Blue arrow oxygen inlet, red carbon dioxide outlet. Pulmonary anatomy. Vector illustration Stock Vector14 Jul 2023 Inhalateur plastique pour inhalation et dégagement des voies respiratoires14 Jul 2023
Inhalateur plastique pour inhalation et dégagement des voies respiratoires14 Jul 2023 Inhalation Manufacturers, Inhaled Drug Delivery14 Jul 2023
Inhalation Manufacturers, Inhaled Drug Delivery14 Jul 2023 Inhalation Solutions - PARI14 Jul 2023
Inhalation Solutions - PARI14 Jul 2023 Smoke inhalation injury: Nursing process (ADPIE) - Osmosis Video Library14 Jul 2023
Smoke inhalation injury: Nursing process (ADPIE) - Osmosis Video Library14 Jul 2023 Chambre d'inhalation 9 mois à 6 ans Anycare14 Jul 2023
Chambre d'inhalation 9 mois à 6 ans Anycare14 Jul 2023
Tu pourrais aussi aimer
 Tortilla espagnole - la Recette de Potimarron14 Jul 2023
Tortilla espagnole - la Recette de Potimarron14 Jul 2023 Dessin Renard Royalty-Free Images, Stock Photos & Pictures14 Jul 2023
Dessin Renard Royalty-Free Images, Stock Photos & Pictures14 Jul 2023 Chausson bébé en cuir souple unis - Noisette14 Jul 2023
Chausson bébé en cuir souple unis - Noisette14 Jul 2023 Gant de jardin cuir Gants pour Professionnels14 Jul 2023
Gant de jardin cuir Gants pour Professionnels14 Jul 2023 Nike Swoosh Sport Tipped headband (6 units) RUNKD online running store14 Jul 2023
Nike Swoosh Sport Tipped headband (6 units) RUNKD online running store14 Jul 2023- Force Glass Original iPhone 12 Pro Max Protège-Ecran en Verre14 Jul 2023
- Aquabeads® Jeu de bricolage enfant perles mallette d'artiste Deluxe14 Jul 2023
 Nike Basket-ball · Sports · El Corte Inglés14 Jul 2023
Nike Basket-ball · Sports · El Corte Inglés14 Jul 2023 Tutoriel pour réaliser une boîte Boite rangement tissu, Cartonnage boite, Boîte en carton tuto14 Jul 2023
Tutoriel pour réaliser une boîte Boite rangement tissu, Cartonnage boite, Boîte en carton tuto14 Jul 2023 Réservoir à eau Krups Dolce Gusto Circolo - Cafetière - 445633814 Jul 2023
Réservoir à eau Krups Dolce Gusto Circolo - Cafetière - 445633814 Jul 2023


