Why Is Water a Polar Molecule?
Par un écrivain mystérieux
Last updated 03 juillet 2024
:max_bytes(150000):strip_icc()/GettyImages-1041588324-5c3cf475c9e77c0001d63bca-5c3f692fc9e77c0001d9a10f.jpg)
Water is a polar molecule because the electrons are unevenly distributed. Since the molecule is polar, water is a polar solvent, also.

How polarity makes water behave strangely - Christina Kleinberg
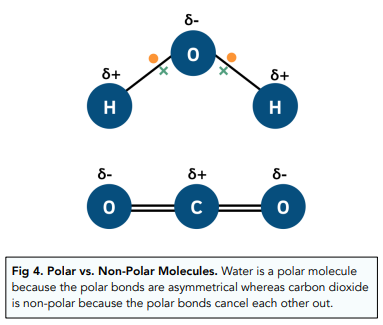
Bonding - Bond Polarity (A-Level Chemistry) - Study Mind
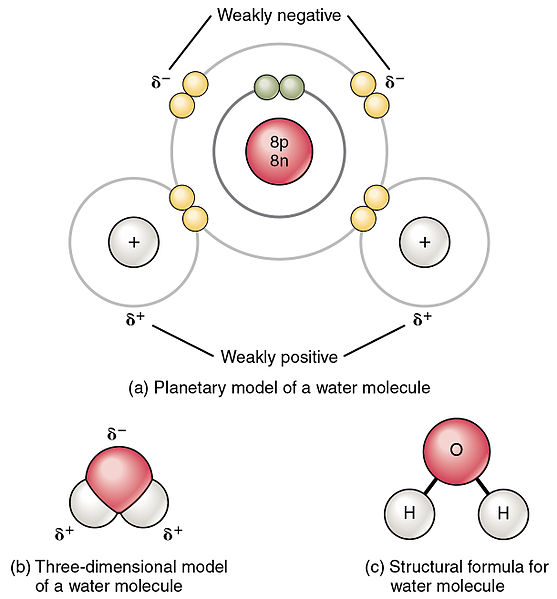
Polar and Non polar molecules - VCE CHEMISTRY PREVIOUS STUDY DESIGN- 2016

Amna's Personal Site

WHY WATER MOLECULE IS POLAR IN NATURE, NCERT, CHEMISTRY
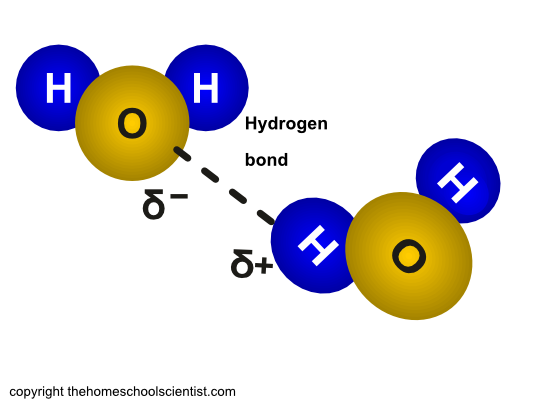
6 Quick Activities for Testing the Properties of Water
Water A Polar Molecule on Vimeo

What is meant by polar water molecules? - Quora
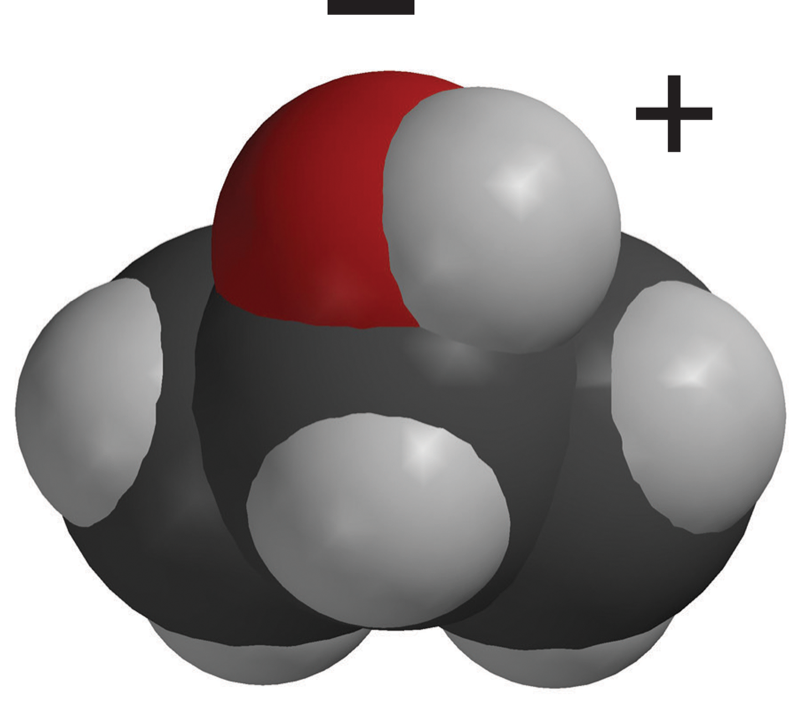
Lesson 5.1: Water is a Polar Molecule - American Chemical Society

Are Polar Molecules Hydrophobic or Hydrophilic?

Properties of Water Polar molecule Cohesion and adhesion High specific heat Density – greatest at 4 o C Universal solvent of life. - ppt download
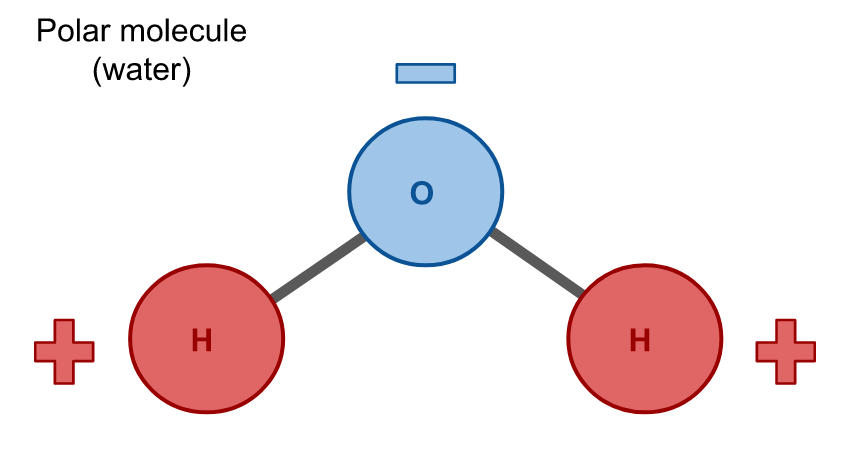
Hydrophobicity

Lesson Explainer: Polar and Nonpolar Solvents

Types of Covalent Bonds: Polar and Nonpolar
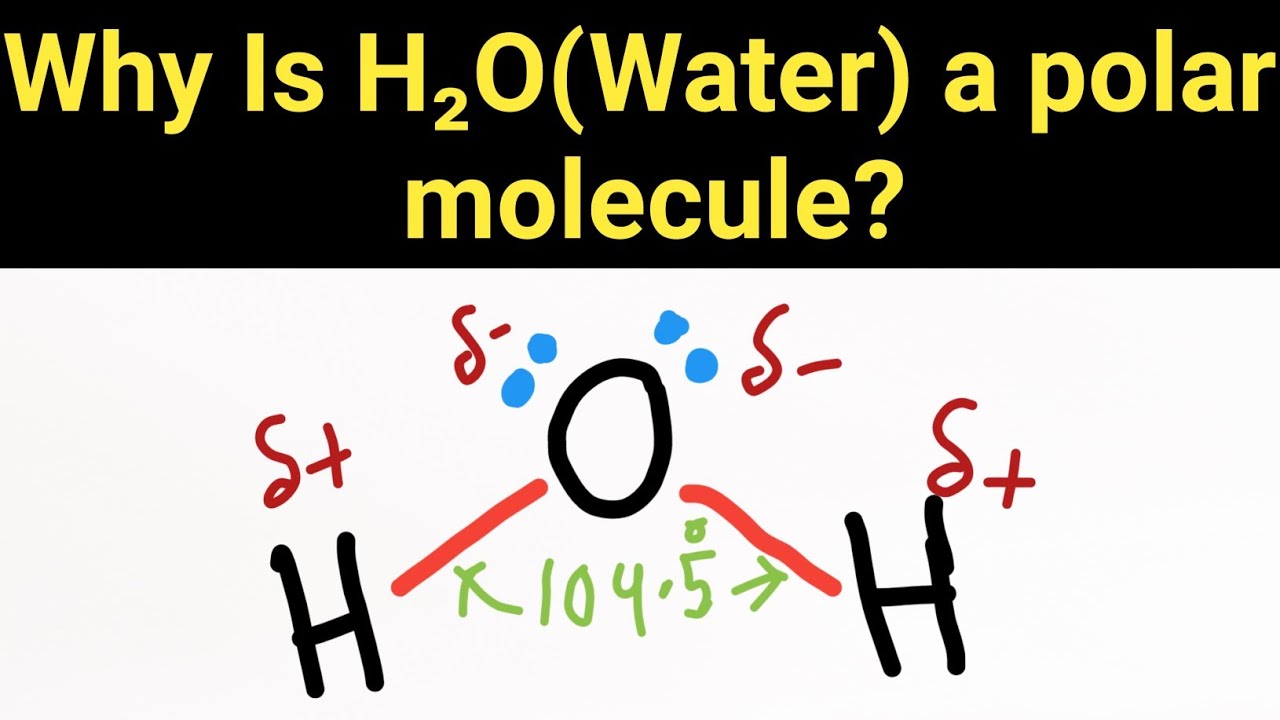
Why is H2O (Water) a polar molecule?
Recommandé pour vous
 Water, Definition, Chemical Formula, Structure, Molecule, & Facts14 Jul 2023
Water, Definition, Chemical Formula, Structure, Molecule, & Facts14 Jul 2023:max_bytes(150000):strip_icc()/mineral-water-8cc11cec12c7471bac9378fa9757c83f.jpg) Health Benefits of Water14 Jul 2023
Health Benefits of Water14 Jul 2023 Biological Roles of Water: Why is water necessary for life? - Science in the News14 Jul 2023
Biological Roles of Water: Why is water necessary for life? - Science in the News14 Jul 2023 A simple method for destroying 'forever chemicals' and making water safe14 Jul 2023
A simple method for destroying 'forever chemicals' and making water safe14 Jul 2023 Bottled water contains alarming amount of nanoplastics: What to know14 Jul 2023
Bottled water contains alarming amount of nanoplastics: What to know14 Jul 2023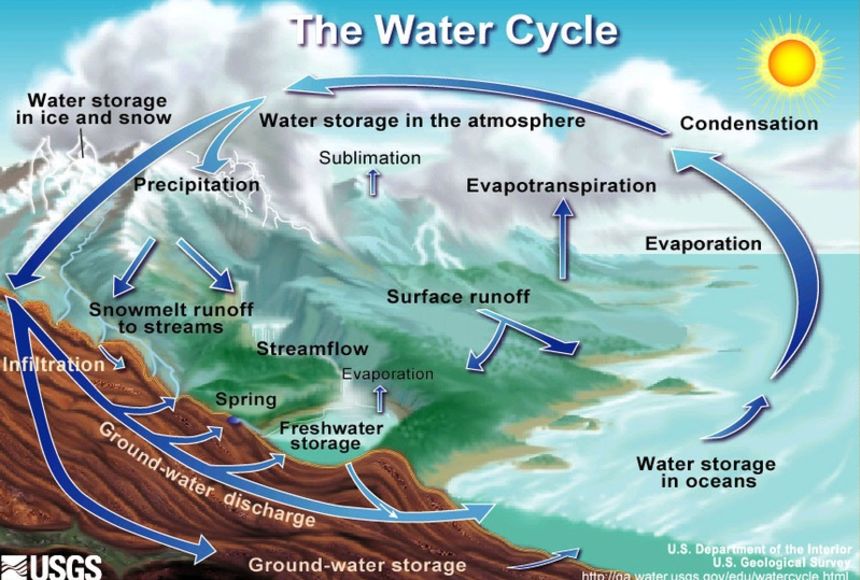 Water Cycle14 Jul 2023
Water Cycle14 Jul 2023 To reduce carbon emissions, just add water14 Jul 2023
To reduce carbon emissions, just add water14 Jul 2023 Is tap water safe to drink? - Reviewed14 Jul 2023
Is tap water safe to drink? - Reviewed14 Jul 2023 Nature's Spring Purified Drinking Water 1 Liter – Nature's Spring Water (PSWRI)14 Jul 2023
Nature's Spring Purified Drinking Water 1 Liter – Nature's Spring Water (PSWRI)14 Jul 2023 Water (H20) - States, Properties & Uses, Water Cycle, Chemistry14 Jul 2023
Water (H20) - States, Properties & Uses, Water Cycle, Chemistry14 Jul 2023
Tu pourrais aussi aimer
 Dame-jeanne : 120 photos inspirantes pour votre déco !14 Jul 2023
Dame-jeanne : 120 photos inspirantes pour votre déco !14 Jul 2023 SYRUP FOR SODASTREAM TORONTO – Fizzy Delivery14 Jul 2023
SYRUP FOR SODASTREAM TORONTO – Fizzy Delivery14 Jul 2023 Stylet Tablette Tactile, 2 en 1 Stylet Tactile Pointe Fine avec14 Jul 2023
Stylet Tablette Tactile, 2 en 1 Stylet Tactile Pointe Fine avec14 Jul 2023 Drôle de danse à énergie solaire Fleur Accessoires de voiture Décoration intérieure automatique14 Jul 2023
Drôle de danse à énergie solaire Fleur Accessoires de voiture Décoration intérieure automatique14 Jul 2023 FORMULER S MINI, BOITIER ANDROID14 Jul 2023
FORMULER S MINI, BOITIER ANDROID14 Jul 2023 Plante, lève-toi et pousse - Sciences et Avenir14 Jul 2023
Plante, lève-toi et pousse - Sciences et Avenir14 Jul 2023 Meuble rangement. Cube meuble rangement dressing, bureau : rangement-easybox14 Jul 2023
Meuble rangement. Cube meuble rangement dressing, bureau : rangement-easybox14 Jul 2023 Interrupteur de commande leve vitre electrique - Cdiscount14 Jul 2023
Interrupteur de commande leve vitre electrique - Cdiscount14 Jul 2023 Figurine 25 cm Groot - Funko Pop - N°1242 Funko : King Jouet, Figurines Funko - Jeux d'imitation & Mondes imaginaires14 Jul 2023
Figurine 25 cm Groot - Funko Pop - N°1242 Funko : King Jouet, Figurines Funko - Jeux d'imitation & Mondes imaginaires14 Jul 2023 Oberthur – Page 3 – Papeterie du Dôme14 Jul 2023
Oberthur – Page 3 – Papeterie du Dôme14 Jul 2023