IQ OQ PQ, Process Validation, Equipment Validation
Par un écrivain mystérieux
Last updated 06 juillet 2024
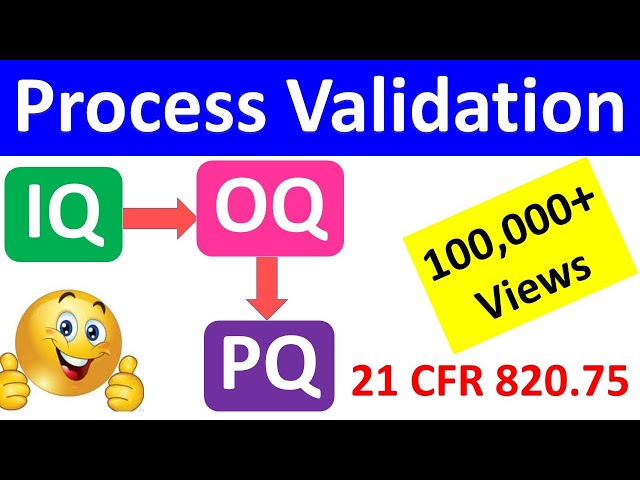
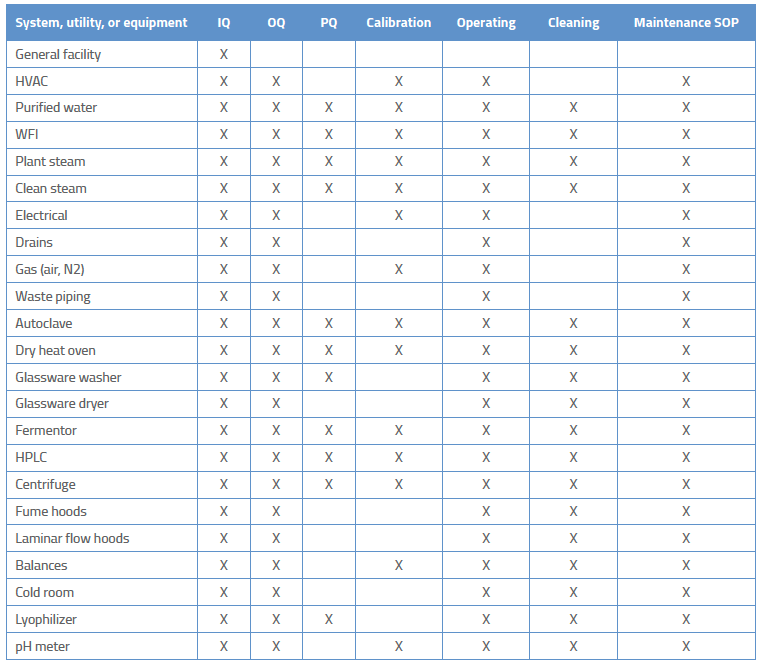
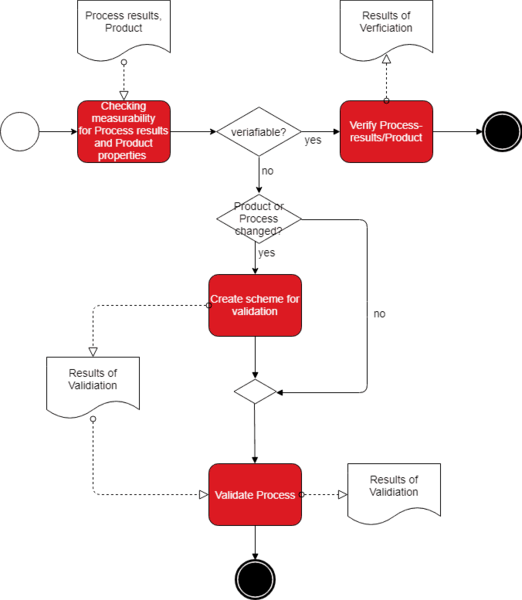
IQ OQ PQ are 3 pillars of Process Validation. IQ stands for Installation Qualification. OQ is Operational Qualification and PQ is Performance Qualification.

IQ, OQ, PQ - A Validation Process in the Medtech Industry - Elos Medtech

Equipment validation
Validation Protocols - Reports Procedure

IQ, OQ, PQ - A Guide to Process Validation by IZiel Group - Issuu

IQ, OQ, and PQ Validation: What You Need to Know
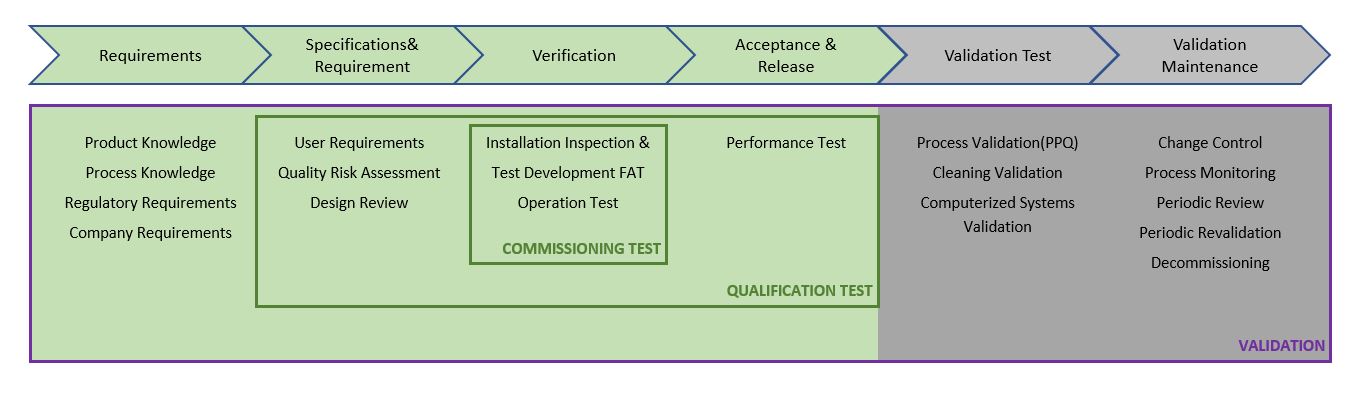
IQ, OQ, and PQ in the Pharmaceutical Industry – No deviation
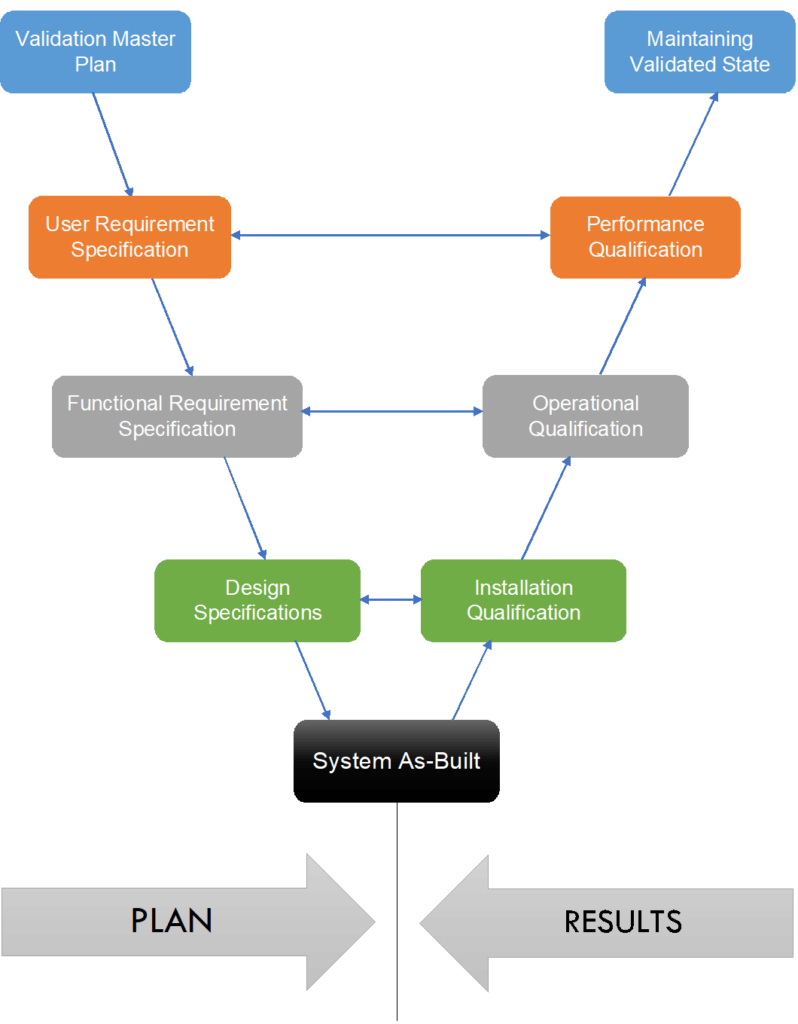
Process Validation: The Essential Guide to Ensuring Product Quality and Compliance - Pharma GxP
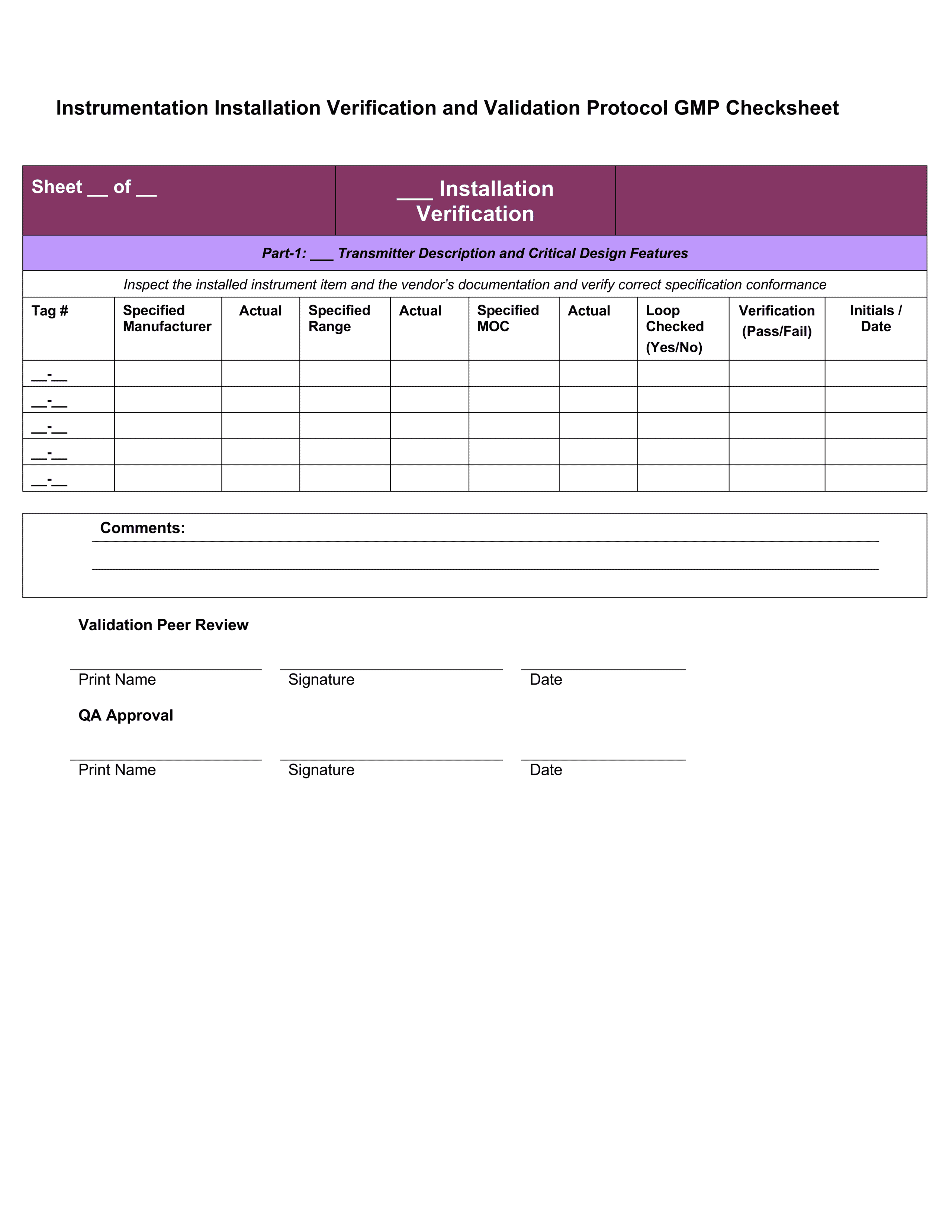
IQ OQ PQ Templates - Download 4 Professional Templates
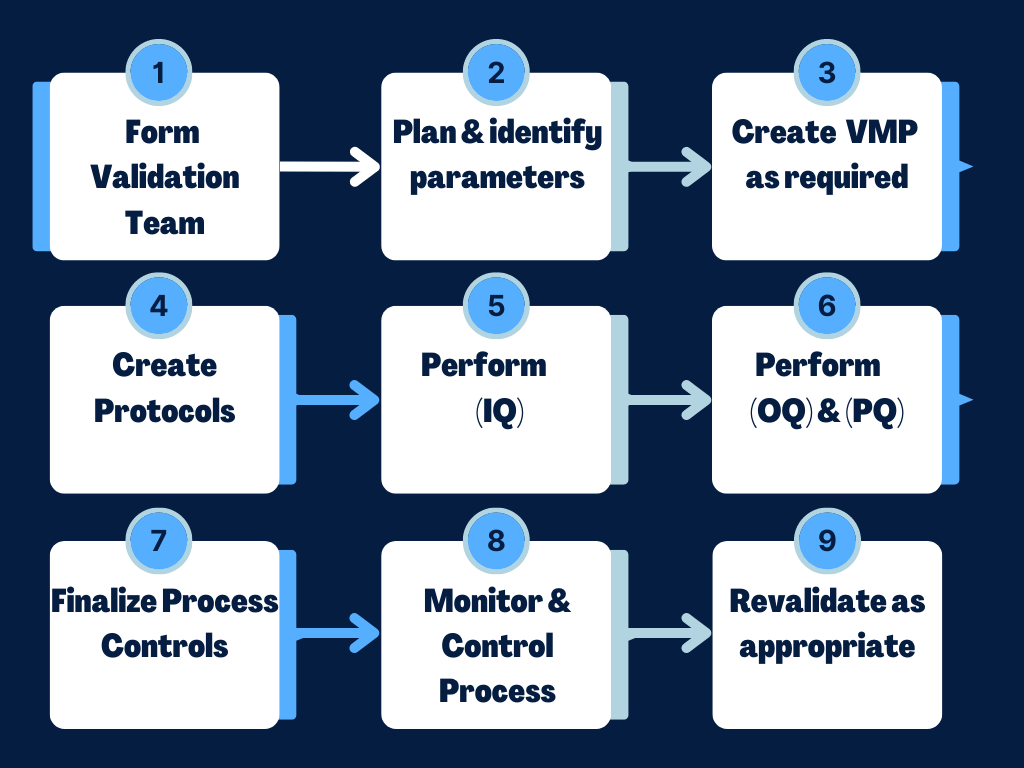
Fast Track ISO 13485 Process Validation Explained for your Medical Device
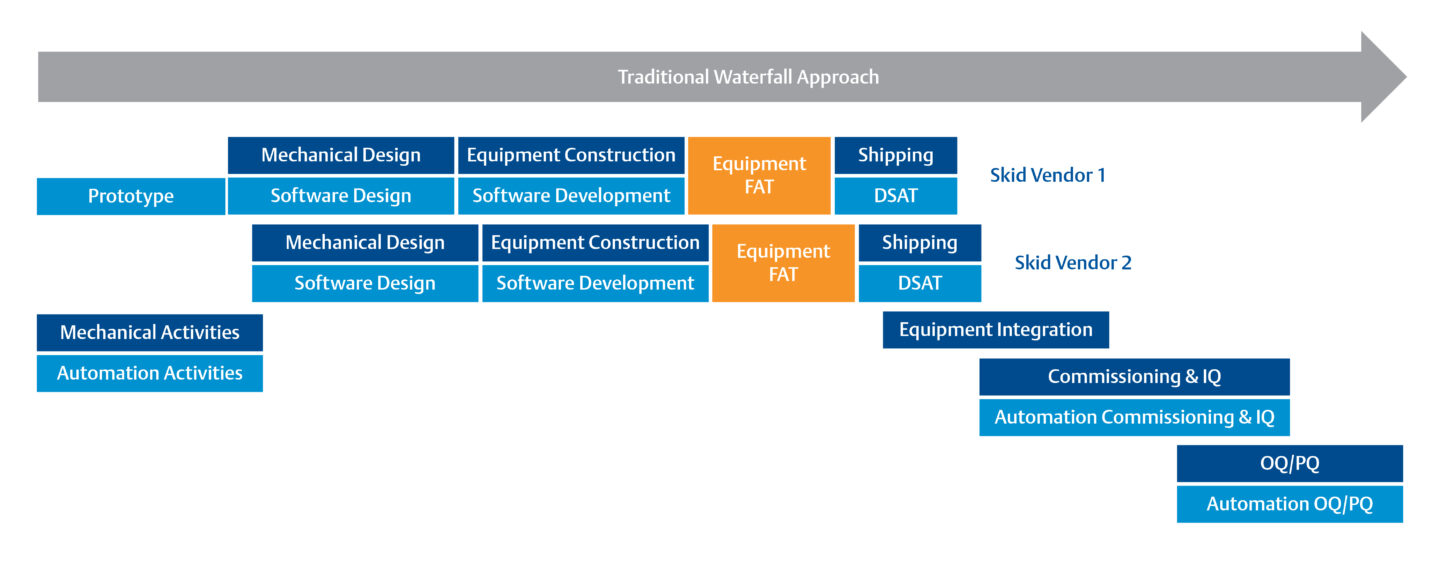
Shorten the Equipment Validation Process to Bring Projects Online Faster
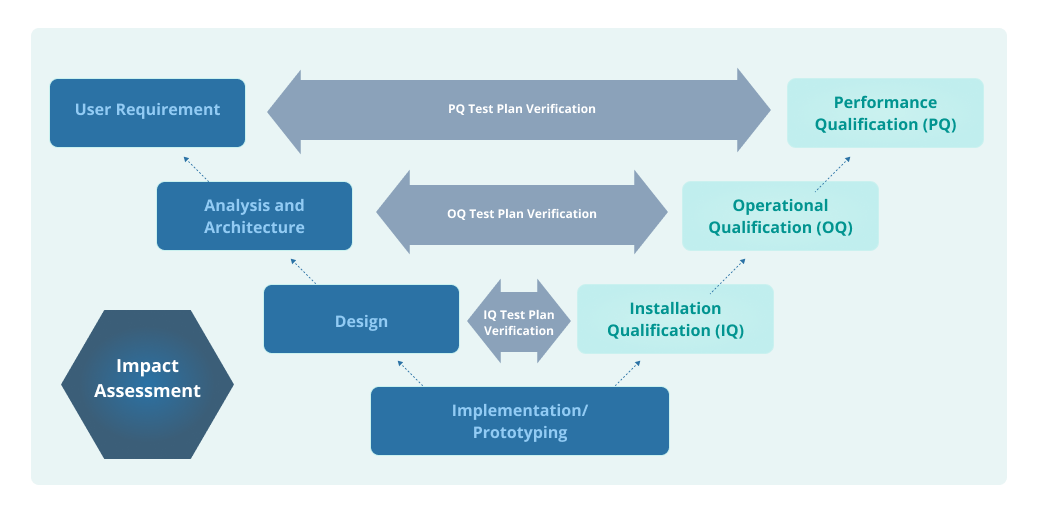
A Guide to IQ, OQ, and PQ in FDA-Regulated Industries

What Are IQ OQ PQ, The 3 Q's Of Software Validation Process
Overview of Medical Device Process Validation: IQ, OQ, and PQ – Oriel STAT A MATRIX – ELIQUENT Life Sciences Blog
Process Validation: Definition & Examples
Recommandé pour vous
 Aurez-vous assez de PQ pour tenir pendant le confinement ?14 Jul 2023
Aurez-vous assez de PQ pour tenir pendant le confinement ?14 Jul 2023 Performance Qualification (PQ) Definition14 Jul 2023
Performance Qualification (PQ) Definition14 Jul 2023 Le PQ se mange aux Attaques14 Jul 2023
Le PQ se mange aux Attaques14 Jul 2023 Pq logo hi-res stock photography and images - Alamy14 Jul 2023
Pq logo hi-res stock photography and images - Alamy14 Jul 2023 Le PQ veut s'attaquer au temps d'écran14 Jul 2023
Le PQ veut s'attaquer au temps d'écran14 Jul 2023 PQ Systems14 Jul 2023
PQ Systems14 Jul 2023- Air PQ14 Jul 2023
 P. Q. Phan: Current: Faculty: Jacobs School of Music: Indiana14 Jul 2023
P. Q. Phan: Current: Faculty: Jacobs School of Music: Indiana14 Jul 2023 PQ 18 ANS14 Jul 2023
PQ 18 ANS14 Jul 2023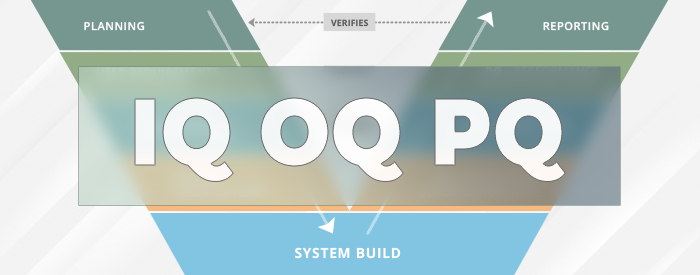 What is IQ OQ PQ in Software Validation?14 Jul 2023
What is IQ OQ PQ in Software Validation?14 Jul 2023
Tu pourrais aussi aimer
 Homgeek Mécanique 24 Heures Minuterie Commutateur IP53 Nominale Programmable Boîte de Commutateur de Minuterie Électrique AC Intervalle de 15 Minutes 96 Fois Marche / Arrêt avec Trou de Montage Étanche à la14 Jul 2023
Homgeek Mécanique 24 Heures Minuterie Commutateur IP53 Nominale Programmable Boîte de Commutateur de Minuterie Électrique AC Intervalle de 15 Minutes 96 Fois Marche / Arrêt avec Trou de Montage Étanche à la14 Jul 2023 Texture De Rendu 3d Du Panneau De Mousse Acoustique Sur Fond Noir, Motif Noir, Motif Sombre, Texture 3d Image de Fond Pour le Téléchargement Gratuit - Pngtree14 Jul 2023
Texture De Rendu 3d Du Panneau De Mousse Acoustique Sur Fond Noir, Motif Noir, Motif Sombre, Texture 3d Image de Fond Pour le Téléchargement Gratuit - Pngtree14 Jul 2023 Iric Material Blue Framed Magnetic Dry Erase Whiteboard (6.5 x 8.25) inch for Home Office School Use | 1 Pack14 Jul 2023
Iric Material Blue Framed Magnetic Dry Erase Whiteboard (6.5 x 8.25) inch for Home Office School Use | 1 Pack14 Jul 2023 Carte Mémoire 16Go Class10 Avec Adaptateur SANDISK - Vente en Ligne14 Jul 2023
Carte Mémoire 16Go Class10 Avec Adaptateur SANDISK - Vente en Ligne14 Jul 2023 MangWany Neiman Kit d'interrupteur à cylindre Start Direction Serrure Couvercle de réservoir avant arrière Côté Porte Compatible avec MASTER 2 MOVANO14 Jul 2023
MangWany Neiman Kit d'interrupteur à cylindre Start Direction Serrure Couvercle de réservoir avant arrière Côté Porte Compatible avec MASTER 2 MOVANO14 Jul 2023 Mega Bloks Halo Universe Red Team Warthog Rescue 324pcs Playset 314 Jul 2023
Mega Bloks Halo Universe Red Team Warthog Rescue 324pcs Playset 314 Jul 2023- Jouets alger - Déguisement: chat noir14 Jul 2023
 Verrine pyramidale plastique PS noire “Taïti” 60 ml 4,5 x 4,5 x 5,5 cm - 30 unités14 Jul 2023
Verrine pyramidale plastique PS noire “Taïti” 60 ml 4,5 x 4,5 x 5,5 cm - 30 unités14 Jul 2023 Solution d'homéopathie contre les coliques14 Jul 2023
Solution d'homéopathie contre les coliques14 Jul 2023 Protege matelas 140 190 epaisseur du matelas 15cm - Cdiscount14 Jul 2023
Protege matelas 140 190 epaisseur du matelas 15cm - Cdiscount14 Jul 2023

