How can graphite and diamond be so different if they are both
Par un écrivain mystérieux
Last updated 05 juillet 2024


TIL over time diamond degrades to graphite as its atoms internally rearrange and relax to a lower energy state. However, at Earth's surface temperature, this process can take millions of years, which
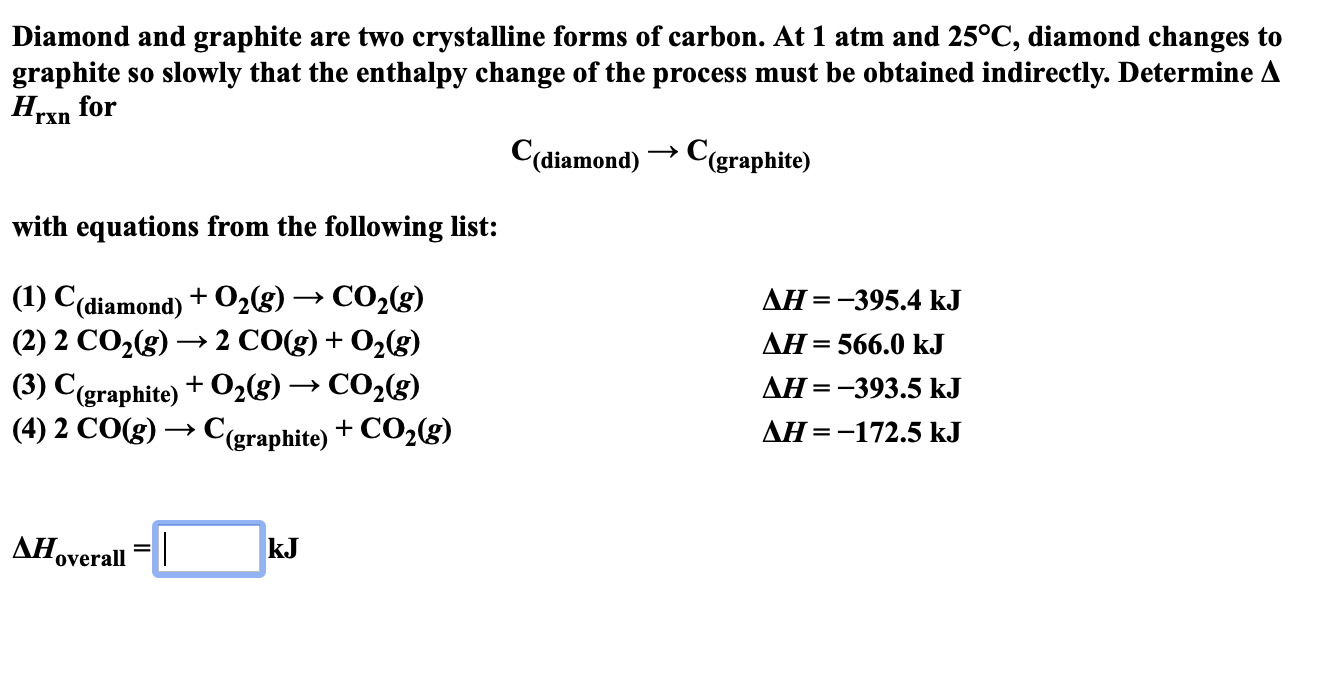
Answered: Diamond and graphite are two…

Different allotropes of carbon viz Graphite, Diamond, Fullerene, and

What Is an Allotrope? Definition and Examples in Chemistry

What is the link between diamond, graphite and buckyballs?
1:50 explain how the structures of diamond, graphite and C60 fullerene influence their physical properties, including electrical conductivity and hardness - TutorMyself Chemistry

Allotropes of Carbon, Definition, Forms & Properties - Lesson

UNM Foundation Engineering - Do you know that diamond and graphite are made from the same element? Source: . . . . . . #WeAreUoN #UoNMalaysia #UNM #nottinghammalaysia #nottinghamuniversity
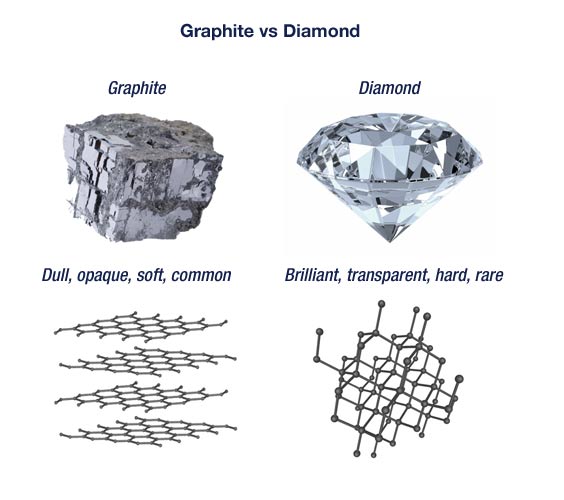
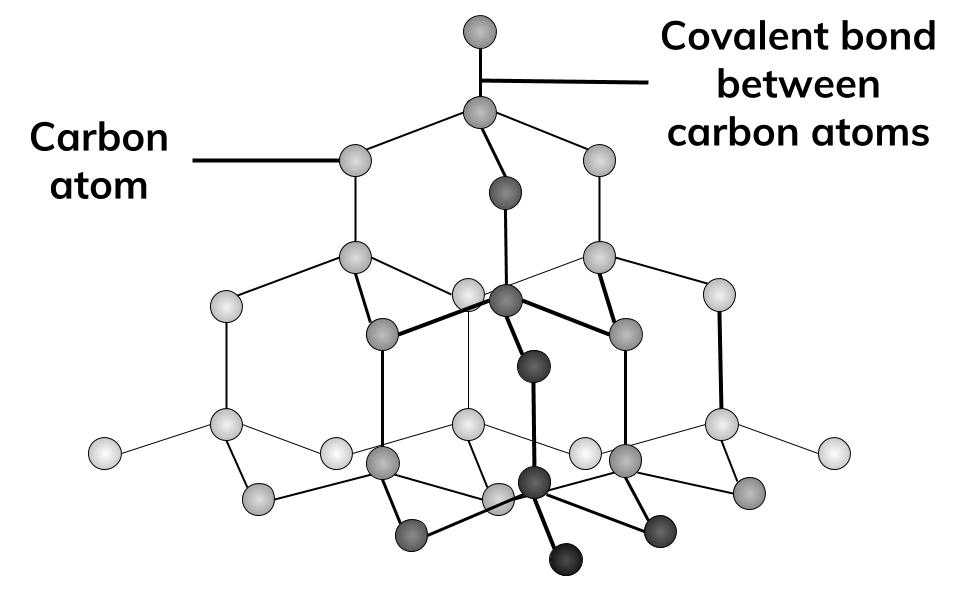
butterfly110 on X: How can graphite and diamond be so different if they are both composed of pure carbon? #Bio110Fall18 @NutmegSomething / X
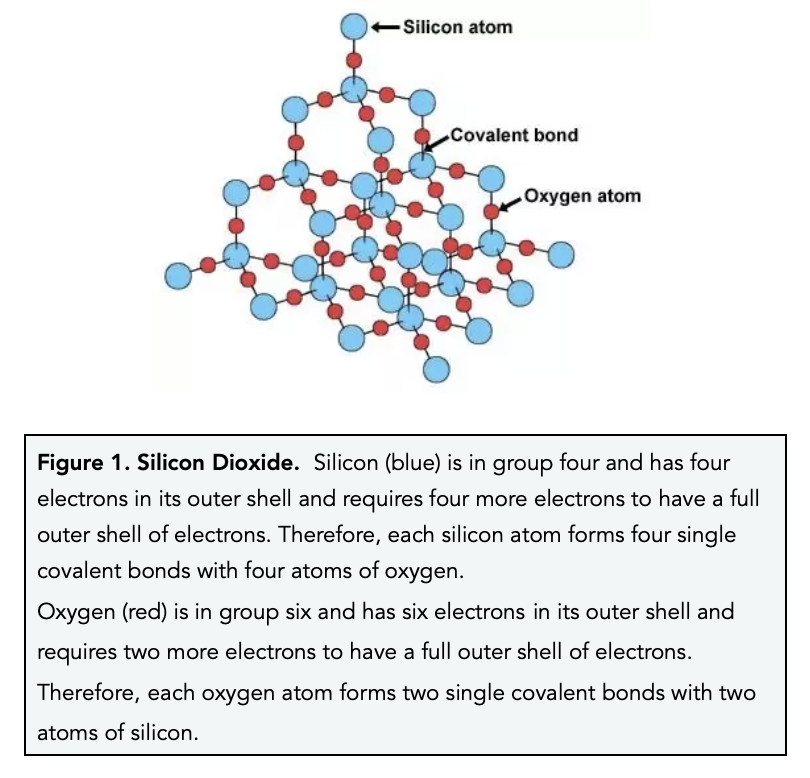
Silicon Dioxide, Diamond & Graphite (GCSE Chemistry) - Study Mind
Recommandé pour vous
 Pitt Graphite Matte Tin of 8 Pencils and Accessories – Faber14 Jul 2023
Pitt Graphite Matte Tin of 8 Pencils and Accessories – Faber14 Jul 2023 Is There Enough Graphite to be Mined for the Electric Car Market?14 Jul 2023
Is There Enough Graphite to be Mined for the Electric Car Market?14 Jul 2023 China ups critical minerals heat with graphite controls14 Jul 2023
China ups critical minerals heat with graphite controls14 Jul 2023 GRAPHITE RESISTORS14 Jul 2023
GRAPHITE RESISTORS14 Jul 2023- Graphite Earth Sciences Museum14 Jul 2023
 Explain the structure of Graphite.14 Jul 2023
Explain the structure of Graphite.14 Jul 2023 China restricts exports of graphite as it escalates a global tech14 Jul 2023
China restricts exports of graphite as it escalates a global tech14 Jul 2023- What Are the Uses of Graphite?14 Jul 2023
![Graphite [SubsTech]](https://www.substech.com/dokuwiki/lib/exe/fetch.php?w=&h=&cache=cache&media=graphite_structure.png) Graphite [SubsTech]14 Jul 2023
Graphite [SubsTech]14 Jul 2023 Graphite14 Jul 2023
Graphite14 Jul 2023
Tu pourrais aussi aimer
 Infinity Deathmatch: TAG Raid14 Jul 2023
Infinity Deathmatch: TAG Raid14 Jul 2023 Béquille vélo repliable sur un coté14 Jul 2023
Béquille vélo repliable sur un coté14 Jul 2023 Types de machines à café : Comment choisir ? - Cuisimark Equipement14 Jul 2023
Types de machines à café : Comment choisir ? - Cuisimark Equipement14 Jul 2023- Blackfox - Chaussure femme jardin14 Jul 2023
 MIKAFEN Gigoteuse bébé à manches longues pour l'hiver - Pour bébé - Motif chien - En coton - Unisexe - Longueur : 100 cm - 3-4 ans - Gris : : Mode14 Jul 2023
MIKAFEN Gigoteuse bébé à manches longues pour l'hiver - Pour bébé - Motif chien - En coton - Unisexe - Longueur : 100 cm - 3-4 ans - Gris : : Mode14 Jul 2023 Tablette d'écriture de cartes flash parlantes, cartes flash parlantes de poche14 Jul 2023
Tablette d'écriture de cartes flash parlantes, cartes flash parlantes de poche14 Jul 2023 Pâte de Curry Rouge - Paris Store14 Jul 2023
Pâte de Curry Rouge - Paris Store14 Jul 2023 Corning™ Bac à glace rectangulaire avec couvercle, Midi 4 l Bleu ; 4 L ; Seau à glace avec couvercle, Midi Corning™ Bac à glace rectangulaire avec couvercle, Midi 4 l14 Jul 2023
Corning™ Bac à glace rectangulaire avec couvercle, Midi 4 l Bleu ; 4 L ; Seau à glace avec couvercle, Midi Corning™ Bac à glace rectangulaire avec couvercle, Midi 4 l14 Jul 2023 How to Use Python: Your First Steps – Real Python14 Jul 2023
How to Use Python: Your First Steps – Real Python14 Jul 2023 KALIGEN® ✮ Piege a Taupe ✮, Pack de 514 Jul 2023
KALIGEN® ✮ Piege a Taupe ✮, Pack de 514 Jul 2023


